Innovation in medical device manufacturing has generated some of the most exciting inventions of the past century. While medical devices have changed considerably, the pioneering spirit lives on and benefits from the increasing pace of technological change, resulting in some fascinating recent developments.
The X-ray allowed us to see deep inside the body without the need for intrusive and often risky surgical procedures. In the past 50 years, we have begun to see the impact that computers have had on healthcare, monitoring patients 24/7, for example, or producing drugs on a mass-scale so that effective pain relief is available and affordable to everyone.
An example of the latest innovations is an innovative drug delivery system developed by Dawson Shanahan, involving the design and production of specialized component parts. It has applied the precision engineering expertise it acquired working in heavier industries to great effect with medical device technology.
Many people still perceive engineering as an oily, harsh and dirty undertaking, wholly apart from the clean, sterile setting of medical and pharmaceutical science. In reality, almost all medical equipment and devices consist of engineered parts. Indeed, even plastic parts have to be engineered, with some being produced by injection molding, pressure or vacuum forming, but many being formed by more traditional methods, such as milling and turning. Everything from syringes and surgical instruments to parts like vascular stents that are designed to be implanted in patients, are engineered for manufacturing.
Dawson Shanahan is pioneering the use of cold forming to produce high-precision, customized components for the pharmaceutical industry. In contrast to conventional component manufacturing methods, precision cold forming can be used to produce extremely high quality components with superior mechanical characteristics and a better surface finish, with considerably less scrap. In many instances, component costs can be reduced by up to 70%, while lead times can be cut by a similar amount.
Cold Forming in Action
Essentially, cold forming is the process of producing components at low, usually ambient temperatures without removing any material, where billets of advanced engineering metals, such as copper, brass, aluminum or steel alloys, are extruded under pressure in a specially designed die set. A simple blank, which has been sawn or cropped from a round bar or wire, or a cold headed pre-form, is placed within a die and a punch is applied to the blank. As a result of the force — typically anything up to 2,000 tons — the blank takes on the form of the punch and the die.
As it is performed at ambient temperatures, cold forming is a far quicker process than more conventional options, allowing manufacturers to achieve much shorter production processes. In turn, this means that components can be made to order extremely quickly, cutting lead times and the need to store high volumes of spare parts onsite. Production cycle times can be cut still further on multi-station machinery, which can be particularly useful in large production runs.
Aside from tangible cost savings, cold forming makes for superior quality products by plasticizing metals along their grain boundaries, rather than cutting across, thus producing parts with extremely low levels of stress deformation and high levels of mechanical integrity, resulting in far greater performance and reliability.
Furthermore, cold forming offers outstanding levels of definition, even on parts with complex contours. Typically, dimensional tolerances can be to within plus or minus two microns, with the added benefit of extremely fine surface finishes, which in many cases, require no further machining or polishing.
Additionally, parts undergo work hardening during the cold forming process, improving their machinability and durability still further. Work hardening dislocates the structure of the metal in a way that prevents further dislocations, resulting in a stronger component. As this increase in strength is comparable to that of heat treating, it can be more cost effective to cold work a less costly and weaker metal than to hot work a more expensive metal, particularly where a precision finish is required.
The cold forming process also makes it possible to produce component parts with a superior finish, both internally and on the surface. Accurate internal profiles and complex external profiles are possible, enabling precision parts to be manufactured that can have a significant impact on the performance of the equipment in which they are used. Furthermore, there is almost no limit to the shape, size or complexity of the metal components that can be produced using cold forming. Simple cold headed parts or highly complex cold formed and finished machined components can be produced for a diverse range of applications.
Accelerating Development
In recent years, the accelerating pace of medical equipment development has wrought dramatic improvements in areas such as body scanning and drug delivery. Just last year, Dawson Shanahan supplied critical components for an innovative, needle-free drug delivery system introduced by a partner, Zogenix, a specialty pharmaceutical company.
Using precision cold forming and engineering techniques Dawson Shanahan produced a highly efficient system that helps reduce patient stress. This case study illustrates that the know-how of an expert precision engineering firm and a collaborative partner can combine for innovative, really groundbreaking, results.
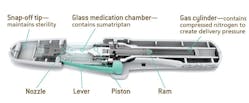
Zogenix was founded with the goal of enhancing and differentiating medicines using new technologies to relieve the suffering of people with CNS and pain disorders. It’s principal product, SUMAVEL DosePro (sumatriptan injection) system, is a drug-device combination that enables needle-free delivery of subcutaneous sumatriptan for the acute treatment of migraines and cluster headaches.
The company has been working with Dawson Shanahan to develop this particular product since its inception. Zogenix had heard of the benefits of cold forming, in terms of its product quality and manufacturing cost savings, and approached Dawson Shanahan to produce one component of the DosePro device by cold forming. They were so impressed with the innovative solution that they then asked for a second component, again to demanding tolerances.
Using its advanced cold forming processes, Dawson Shanahan achieved a specially engineered part with a highly accurate pressure and finish that allows the drug to be shot into the system without the need for a needle. A mirror surface on the inside of the piston and the angles in the design are crucial to ensure that the trigger mechanism works effectively.
The developer worked closely with Zogenix to enhance the design of the DosePro system, and suggested that it could manufacture a number of integral elements of the system in-house and within budget. With assistance from Simon West at E-Tech, it set to work creating the customized machining and tooling needed to manufacture one of the components and together developed and tested the parts until an optimum solution was found.
Bill Feinstein, director of Operations Planning and Procurement at Zogenix, explained that the partnership between the two companies had been critical to the successful development of DosePro.
“Throughout the manufacturing process, Dawson Shanahan was extremely collaborative, throwing the necessary resources at challenges to help us find the best solutions,” Feinstein stated. “DosePro is a product based on physics and comprises many parts that all have to work exactly the way they are intended and in harmony together.”
He praised the manufacturer for its vital role in the product development, and indicated that Zogenix would continue to work with Dawson Shanahan for future projects. With such successful developments, it will be no surprise when cold forming is applied more widely for medical device manufacturing, and in other industries.
Mark Jennings is the engineering director at Dawson Shanahan Ltd., a specialty cold forming and machining company producing high-precision, customized designs in copper, aluminum, and ferrous alloys, as well as assembled components. Visit www.dawson-shanahan.co.uk, or contact [email protected] for more information.